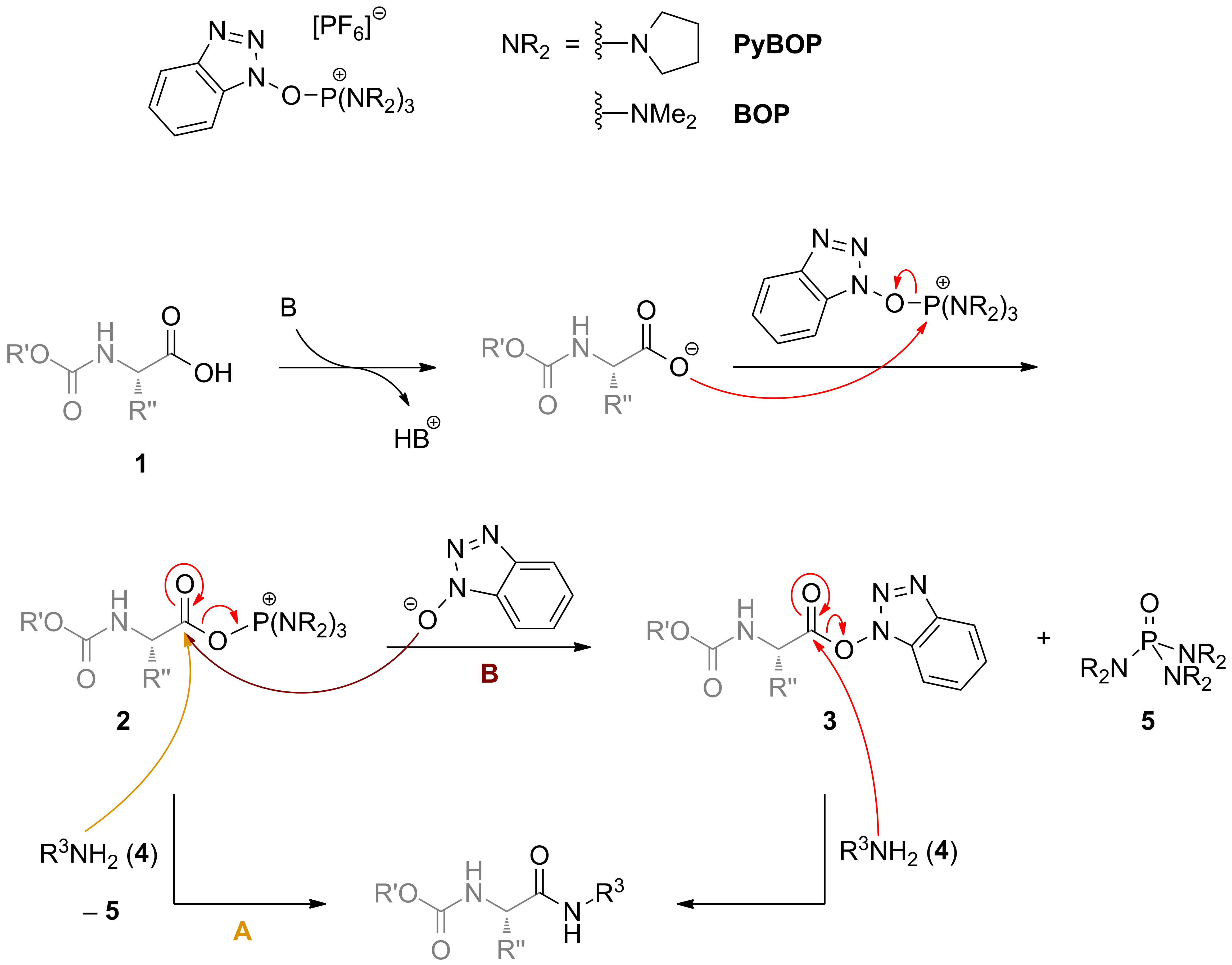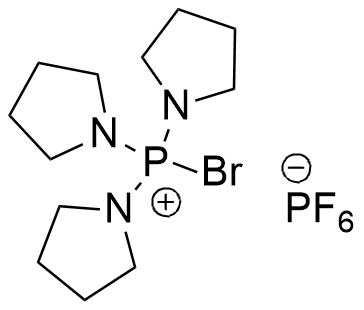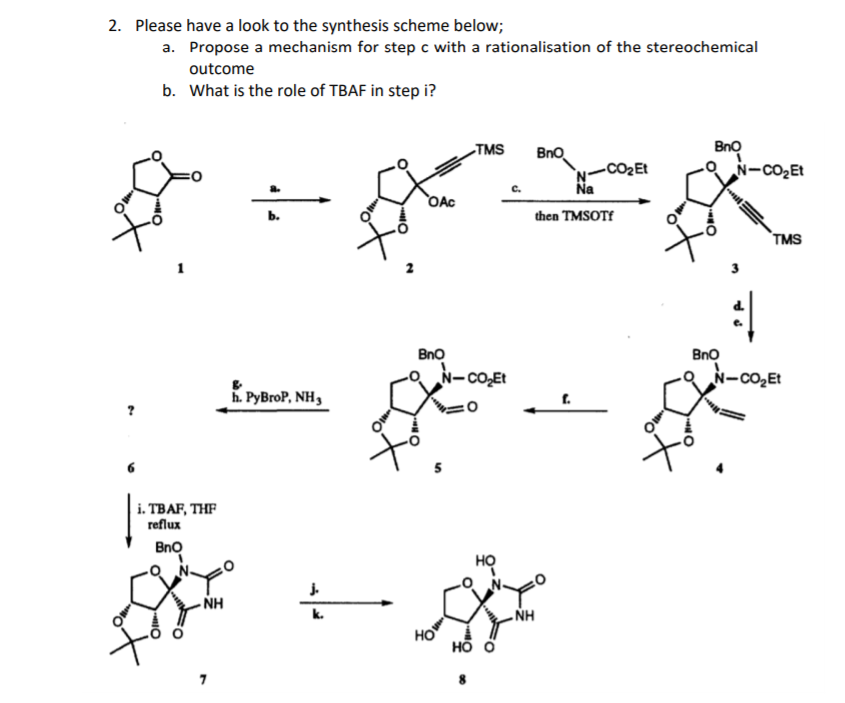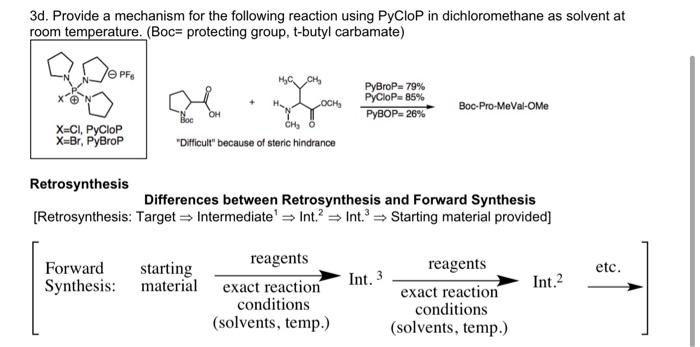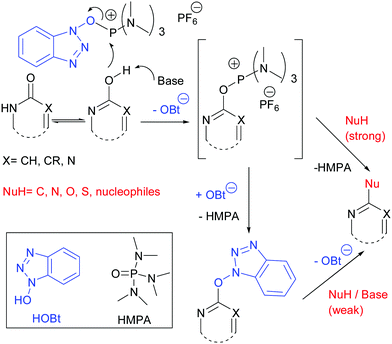
Coupling reaction between electron-rich pyrimidinones and α-amino acids promoted by phosphonium salts - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C1OB05313B

Molecules | Free Full-Text | The Application of Biomass-Based Catalytic Materials in the Synthesis of Cyclic Carbonates from CO2 and Epoxides

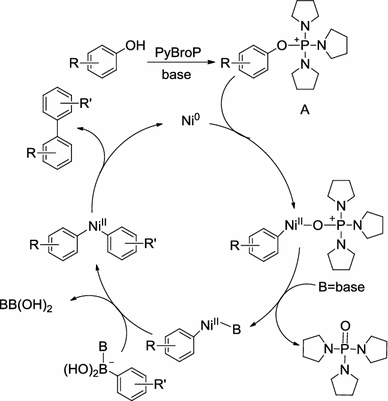
Nickel‐Catalyzed Cross‐Coupling of Phenols and Arylboronic Acids Through an In Situ Phenol Activation Mediated by PyBroP - Chen - 2011 - Chemistry – A European Journal - Wiley Online Library
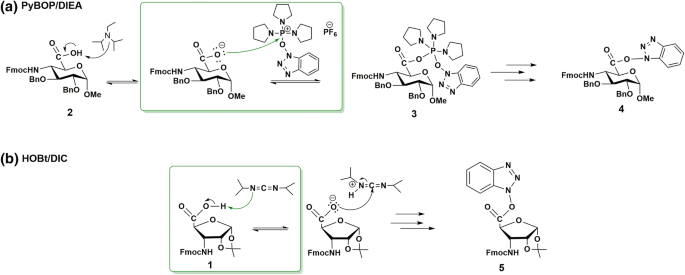
α/β-Chimera peptide synthesis with cyclic β-sugar amino acids: the efficient coupling protocol | SpringerLink

Phosphonium Coupling in the Direct Bond Formations of Tautomerizable Heterocycles via C–OH Bond Activation - Kang - 2009 - European Journal of Organic Chemistry - Wiley Online Library
Recent approaches for C–C bond formation via direct dehydrative coupling strategies - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C2CS35397K

Reaction Mechanism of the Reverse Water–Gas Shift Reaction Using First-Row Middle Transition Metal Catalysts L′M (M = Fe, Mn, Co): A Computational Study | Inorganic Chemistry

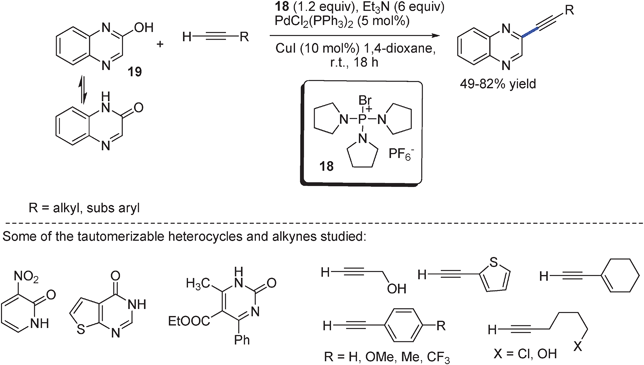
Efficient Pd-catalyzed coupling of tautomerizable heterocycles with terminal alkynes via C-OH bond activation using PyBrOP. - Abstract - Europe PMC
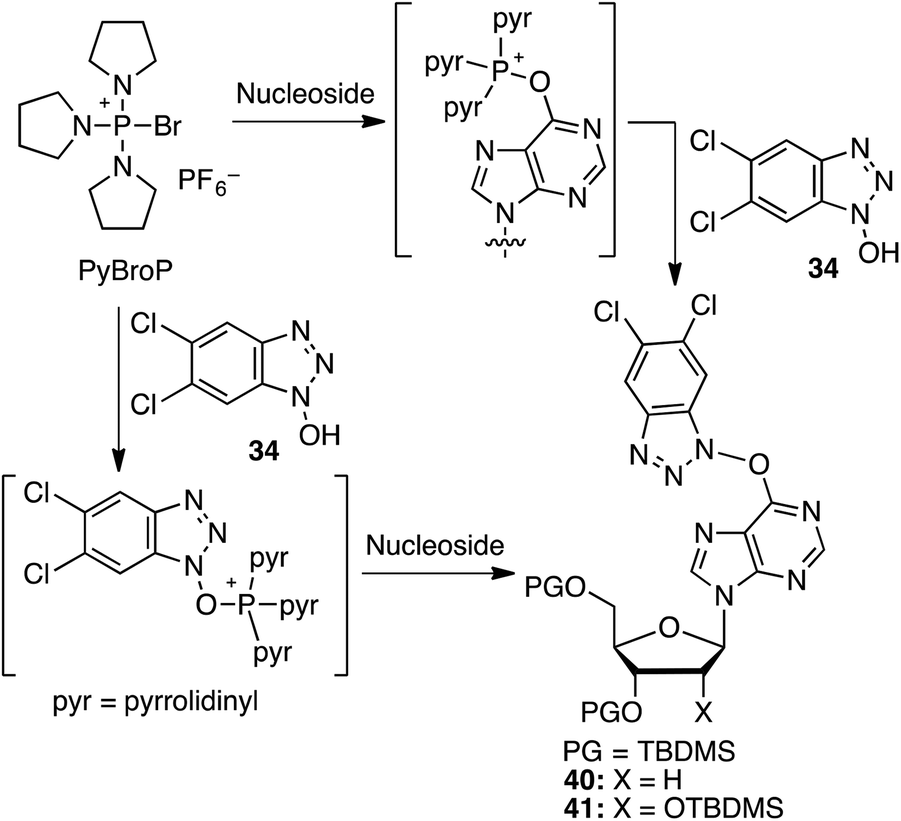
A novel bis(pinacolato)diboron-mediated N–O bond deoxygenative route to C6 benzotriazolyl purine nucleoside derivatives - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C6OB01170E


![Catalysts | Free Full-Text | Recent Advances in Catalytic [3,3]-Sigmatropic Rearrangements | HTML Catalysts | Free Full-Text | Recent Advances in Catalytic [3,3]-Sigmatropic Rearrangements | HTML](https://www.mdpi.com/catalysts/catalysts-12-00227/article_deploy/html/images/catalysts-12-00227-g003-550.jpg)